

















The conjugate acid of HSO4 is H2SO4 . Acid is a compound which gives H+ ion and base is a compound which accepts H+ ion. H2SO4 gives H + ion to form HSO4 hence it is conjugate acid of HSO4 and similarly HSO4 accepts H+ ion to give H2SO4 hence it is conjugate base of H2 SO4. 1.8K views. *Response times vary by subject and question complexity. Median response time is 34 minutes and may be longer for new subjects. Q: 56) What is the concentration, in molarity, of NaI in a solution that contains 7.3 g of NaI (MM = 14... A: If the mass value of any particular solute (dissolved Assuming you're referring to a acid/conjugate base pair, an example would be H2SO4 and HSO4-. A conjugate base is what you get after removing a hydrogen atom from an acid. HSO4- is, in fact, the conjugate base of the acid H2SO4. H2SO4 is an acid so it donates a proton and after donating a proton it will be HSO4-H2SO4 = HSO4- + H+. HSO4- is a base since it has the ability to accept a proton but it is a conjugate base to H2SO4 since it is formed by the H2SO4 after donating a proton. Problem: What is the conjugate base of HSO4- A. HSO4- B. H2SO4 C. SO4 D. HSO4 E. SO42- FREE Expert Solution Show answer. 93% (275 ratings) Problem Details. What is the conjugate base of HSO 4-A. HSO 4-B. H 2 SO 4 C. SO 4 D. HSO 4 E. SO 4 2-Learn this topic by watching Conjugate Acids and Bases Concept Videos. Click here👆to get an answer to your question ️ The conjugate base of H2PO4^- is: Click here👆to get an answer to your question ️ The conjugate base of H2SO4 in the following reaction is: H2SO4 + H2O → H3O^+ + H SO4^- The given species H2SO4 is an acid so it is suppose to donate a proton and after donating a proton it will be HSO4 - H2SO4 <-----> HSO4- + H + Now, HSO4-is a base since it has the ability to accept a proton but it is a conjugate base to H 2 SO 4 since it is formed by the H2SO4 after donating a proton. H 2SO4 sulfuric acid will donate an H + in solution to form H 3O+ hydronium. The remaining H SO− 4 would be the conjugate base of this dissociation. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a (25 oC) HClO 4 ClO 4 – H 2 SO 4 HSO 4 – HCl Cl– HNO 3 NO 3 – H 3 O + H 2 O H 2 CrO 4 HCrO 4 – 1.8 x 10–1 H 2 C 2 O 4 (oxalic acid) HC 2 O 4 – 5.90 x 10–2 [H 2 SO 3] = SO 2 (aq) + H2 O HSO
[index] [3454] [8407] [6040] [9206] [4751] [6510] [1615] [5127] [5757] [2684]
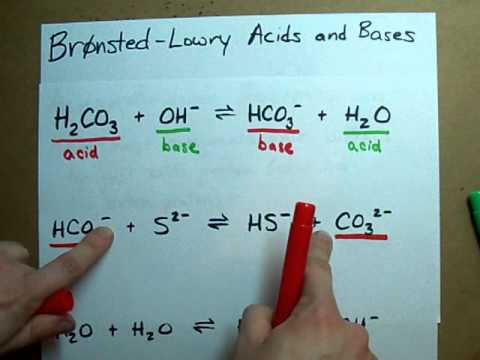
In this video we'll balance the equation KOH + H2SO4 = K2SO4 + H2O and provide the correct coefficients for each compound. To balance KOH + H2SO4 = K2SO4 + ... SO3 +H2O =H2SO4 Balanced Equation Sulfur trioxide ,Water, Sulfuric acid Balanced Equationchemistry crash course,crash course in chemistry,crash course on c... Chemistry: Learn more at: http://www.pathwaystochemistry.com/chemistry-qa/videos/conjugate-acid-base-pairs/ Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com Acids and their conjugate bases, and bases and their conjugate acids. Water has both properties of an acid and a base. The content of this video is designed to accompany the 12th edition of "Chemistry The Central Science" by Brown, Lemay, Bursten, Murphy, and Woodward. The ti... In this video we will look at the equation for H2SO4 + H2O and write the products. There are two reactions we need to consider since both of the hydrogens... Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together...
Copyright © 2024 top.onlinerealmoneytopgames.xyz